Novel Protective Antibody Target Against Crimean Congo Hemorrhagic Fever Virus Identified
A research team led by the United States Army Medical Research Institute of Infectious Diseases, has discovered an important protective antibody target against Crimean-Congo Hemorrhagic Fever virus, or CCHFV. Their work, which appears online today in the journal Nature Communications, could lead to the development of protective antibodies for infected patients.
CCHF is considered a priority pathogen by the World Health Organization, or WHO, as it is an emerging zoonotic disease with a propensity to spread. It is also endemic in large portions of the world. CCHF outbreaks have a mortality rate of up to 40 percent. Originally described in Crimea in 1944–1945, and decades later in the Congo, the virus has recently spread to Western Europe through ticks carried by migratory birds. The disease is already endemic in Africa, the Balkans, the Middle East, and some Asian countries. CCHFV is designated as a biosafety level 4 pathogen (the highest level of biocontainment) and is a Category A bioterrorism/biological warfare agent. There is no vaccine to help prevent infection and therapeutics are lacking.
USAMRIID has worked with CCHFV going back several decades. During this work, the institute amassed an extensive collection of mouse monoclonal antibodies against CCHFV. However, suitable animal model systems to study antibody protection against CCHFV were only recently developed, some at USAMRIID. In a previous work, USAMRIID scientists Aura R. Garrison and Joseph W. Golden studied these collections of monoclonal antibodies in newly developed animal systems to identify a novel CCHFV therapeutic antibody target called glycoprotein 38, or GP38. GP38 was previously identified by the US Centers for Disease Control as a viral protein with unknown function.
Here, Garrison and Golden explored the protective efficacy of a CCHFV monoclonal antibody targeting the nucleocapsid protein (NP). The scientists found that the NP targeting antibody protected mice against an otherwise lethal infection. This included providing substantial protection against a strain of CCHFV taken from a lethal human case. This work therefore opens another new direction for immunotherapeutics targeting CCHFV. The group is currently exploring the mechanism of protection of anti-NP antibodies. "We hope to combine antibodies targeting NP with those targeting GP38 to produce a robust immunotherapeutic cocktail that can protect humans," said Garrison.
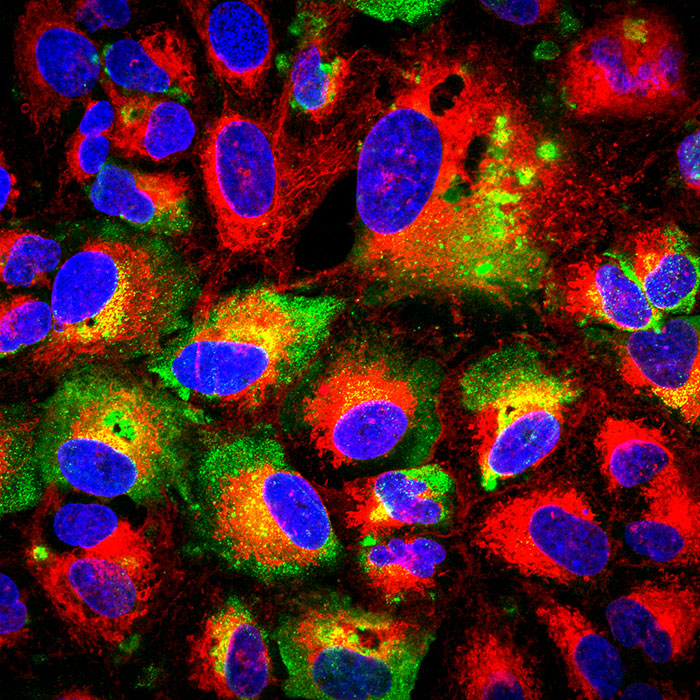
The antibody used for these studies is called MAb-9D5 and prior to this study was used as a laboratory reagent. NP is generally not thought of as a key target for protective antibody because it is buried withing the virus structure and not believed to be expressed in a manner accessible to protective antibodies. The work in this study revealed that NP is present on the surface of infected cells, which may be the reason why the antibody is able to provide protection. The University of California Riverside School of Medicine (led by Scott Pegan) and the US Centers for Disease Control (led by Éric Bergeron) joined in this study and showed that NP targeting antibodies can interact with NP from several CCHFV strains suggesting that this product could protect against most strains of CCHFV circulating throughout the world. "Exposure of hospital workers and overseas military service personnel to CCHFV is a major problem, therefore an antibody-based drug that broadly protects prophylactically against existing strains of CCHFV, while also providing therapeutic protection is ideal," stated Professor Pegan.
The research was funded by grants to Golden and Garrison from the Military Infectious Disease Program. Pegan (University of California Riverside School of Medicine & U.S. Military Academy) and Bergeron (Center for Disease Control) were funded with grants from the National Institutes of Health and the Department of Defense.
Garrison and Golden were joined in the study by Vanessa Moresco, Xiankun Zeng, Curtis Cline, Michael Ward, Keersten Ricks, Scott Olschner, Lisa Cazares, Elif Karaaslan, Collin Fitzpatrick, Éric Bergeron and Scott Pegan.
About the U.S. Army Medical Research Institute of Infectious Diseases:
Since 1969, USAMRIID has provided leading edge medical capabilities to deter and defend against current and emerging biological threat agents. The Institute is the only laboratory in the Department of Defense equipped to safely study highly hazardous viruses requiring maximum containment at Biosafety Level 4. Research conducted at USAMRIID leads to vaccines, drugs, diagnostics, and training programs that protect both Warfighters and civilians. The Institute's unique science and technology base serves not only to address current threats to our Armed Forces but is an essential element in the medical response to any future biological threats that may confront our nation. For more information, visit usamriid.health.mil.