Scientists Develop First Lethal Hamster Model for COVID-19
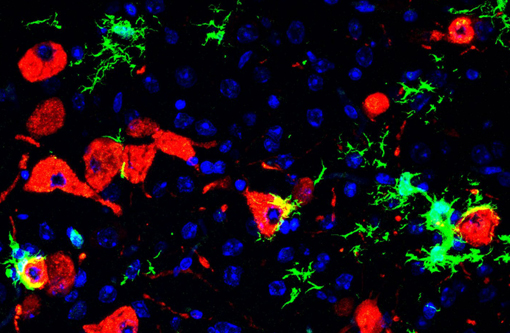
Army scientists, in collaboration with Utah State University, have developed a lethal hamster model for SARS-CoV-2, the virus that causes COVID-19. This model system will aid in understanding the severe disease course of COVID-19–including the virus's interaction with the central nervous system–and will support the advancement of vaccines and therapeutics targeting the virus.
Joseph W. Golden, Ph.D. and colleagues at the U.S. Army Medical Research Institute of Infectious Diseases collaborated with Zhongde Wang, Ph.D. and the USU team to develop the hamster model. Their findings appear in today's online edition of mBio, a journal published by the American Society for Microbiology.
SARS-CoV-2, the novel coronavirus first identified in Wuhan, China in late 2019, has caused a global pandemic resulting in significant morbidity and mortality in humans throughout the world. COVID-19 disease presentation in humans ranges from asymptomatic virus carrier to fatal systemic disease, and historic efforts are underway to develop medical countermeasures. Animal models that mimic the course of human disease are essential for advancing these countermeasures.
Furthermore, understanding how the virus can produce severe, and potentially deadly, disease in a small percentage of the population is an essential aspect of these studies. While SARS-CoV-2 most typically causes a respiratory infection, more evidence suggests that there may also be a neurological aspect of COVD-19 that is poorly understood, according to Golden, the paper's first author.
To explore this avenue, USU's team developed a transgenic hamster genetically engineered to express the human angiotensin converting enzyme 2 (ACE2) gene–a key protein used by SARS-COV-2 to enter cells–under the control of a keratin 18 promoter. This promoter increases the level of ACE2 expression to produce a more acute form of the disease, allowing scientists to study infection of the central nervous system, including the brain and spinal cord.
Hamsters, like humans, are naturally susceptible to infection by SARS-CoV-2, in part because they have similar ACE2 proteins at important virus binding sites, the researchers said. Ordinarily, infected hamsters develop respiratory disease; the virus is controlled by the host and cleared; and the animals survive. In this way, the normal hamster system is similar to human disease, which is typically moderate in nature. However, the K18-hACE2 transgenic animals developed acute disease, including weight loss, lung injury, and brain infection, and ultimately succumbed to the disease.
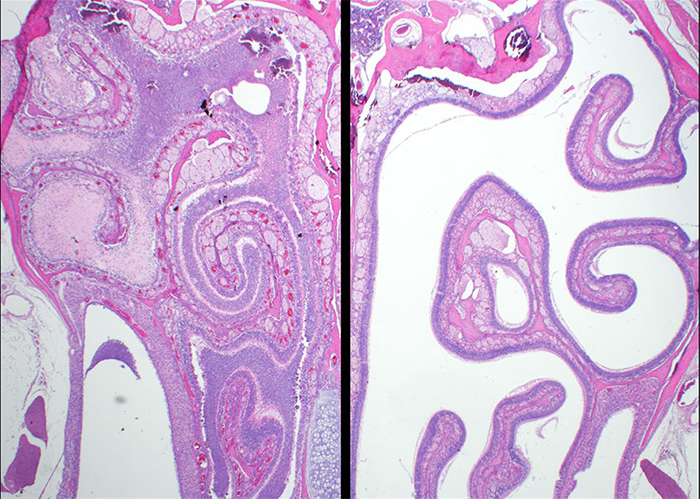
"Working with Utah State University and leveraging their transgenic animal technology, together with USAMRIID's emerging infectious disease research expertise, has allowed us to more comprehensively understand the pathophysiology of SARS-CoV-2," Golden said.
Additionally, the authors found that uninfected control animals in proximity to infected animals also had the virus, indicating that this model system is highly sensitive to SARS-CoV-2 infection and may be ideal for studying viral spread between hosts.
"This hypersensitive hamster model might be particularly useful for variants of concern that cause mild disease in wild type hamsters," said Jay W. Hooper, Ph.D., the paper's senior author.
About the U.S. Army Medical Research Institute of Infectious Diseases:
For over 53 years, USAMRIID has provided leading edge medical capabilities to deter and defend against current and emerging biological threat agents. The Institute is the only laboratory in the Department of Defense equipped to safely study highly hazardous viruses requiring maximum containment at Biosafety Level 4. Research conducted at USAMRIID leads to vaccines, drugs, diagnostics, and training programs that protect both Warfighters and civilians. The Institute's unique science and technology base serves not only to address current threats to our Armed Forces, but is an essential element in the medical response to any future biological threats that may confront our nation. USAMRIID is a subordinate laboratory of the U.S. Army Medical Research and Development Command. For more information, visit usamriid.health.mil.
Reference:
The article, "Hamsters Expressing Human Angiotensin-Converting Enzyme 2 Develop Severe Disease following Exposure to SARS-CoV-2," is available at this link: https://doi.org/10.1128/mBio.02906-21.
Authors:
Joseph W. Golden, Rong Li, Curtis R. Cline, Xiankun Zeng, Eric M. Mucker, Amadeo J. Fuentes-Lao, Kristin W. Ostrowski, Janice A. Williams, Nancy Twenhafel, Neil Davis, Joshua L. Moore, Stephen Stevens, Eugene Blue, Aura R. Garrison, Deanna D. Larson, Rebekah Stewart, Madelyn Kunzler, Yanan Liu, Zhongde Wang, and Jay W. Hooper
Funding:
Funding was provided through the Defense Health Agency; the transgenic hamster lines were established through a National Institutes of Health contract.
 An official website of the United States government
An official website of the United States government
 ) or https:// means you've safely connected to the .mil website. Share sensitive information only on official, secure websites.
) or https:// means you've safely connected to the .mil website. Share sensitive information only on official, secure websites.
